Standards, Policy and Procedures Committee
The Drug Administration Guidelines added to the “New to You–Time to Review” e-mail update is an example of the committee’s ongoing work to communicate changes in a timely manner. It will be fine-tuned, but the committee expects this feature to remain.
Emergency Department and Critical Care Units: A new policy on the use of induced hypothermia is complete. ICU-44 reviews the multiple key components of equipment use, care of the patient, monitoring and re-warming. This policy is time critical and its use will likely increase. Recommendations are welcome as staff gain more experience with this treatment modality. There are two “Arctic Sun” units, one in the CCU and one in the MICU.
The language regarding vaccine administration has changed from “reassessed” to “screening criteria met.” This is to reflect practice and continue to prompt clinical nurses to incorporate immunization into practice prior to transfer, or to communicate it when transferring if the patient has a clinical reason not to have received it prior to the transfer. If the clinical condition does not allow it, the order will need to be re-written as it will “fall-off” EMAR in the transfer session.
The Nursing Admission Form (FOR-19) has changed. There will be three new questions added to the top of the page that contains the Morse Falls Scale. A positive response to any of the questions should trigger the nurse to obtain an order for a physical therapy consult.
The committee members appreciate the feedback and suggestions they received from nurses.
For more information, contact co-chairs Sharon Swan, BSN, RN, CRN, and Cindy Jodoin, MHA, BSN, RN.
Quality, Safety and Care Improvement Committee
The Quality, Safety and Care Improvement Committee together with the Center of Clinical Excellence conducted a workflow review of the transfer process between the ICUs and the intermediate care units. The purpose of the review was to verify the steps of the current process and to highlight those process points that may vary or not be accurate. This information will be used to strengthen the order entry process for transfers and to ensure identification of the receiving physician team upon admission.
The committee also heard a presentation on the recent status of the Hand Hygiene program and brainstormed for other initiatives and actions that would effectively promote and make certain all nursing staff maintain good hand hygiene.
For more information, contact co-chairs Stephanie Capello, RN, and Mary Antonelli, MPH, RN.
Informatics and Clinical Innovations Committee
The Informatics and Clinical Innovations Committee advocates for practice to drive technology to assist the clinical nurse at the bedside and promote patient and staff safety. Current projects include:
eMAR Updates/Enhancements
The committee provided feedback on new screens for the administration of vaccines coming to eMAR at the end of June. When a vaccine is scanned in eMAR at the time of the administration, nurses will be prompted to enter information about the administration (i.e., lot # and expiration date) that is currently documented on the paper nursing assessment. This allows nurses the capability to run a report in eMAR that will display all of the vaccines documented in eMAR either as given or not given. Nurses will continue to fill out the screening portion of the nursing assessment.
Suggestions were given on how to indicate in eMAR that a medication should not be crushed. The committee gave approval of a new icon for display on the To Do List in eMAR indicating that a medication can be IV pushed.
ACD (Acute Care Documentation)
ACD-Notes: The committee discussed ways to help staff manage synthesis/progress note documentation in the ACD application.
ACD-PCA Workflow: The committee is working on identifying PCA documentation responsibilities and work flow. The goal is to create an easy-to-use form in ACD for PCA documentation. The committee agreed that there should be a separate form for PCAs to use that will send information to the electronic flow sheet.
eMAR/Metavision Integration: Review of list of blood products that will need to display on the ACD (Metavision) flow sheet once they are given in eMAR. Products not in eMAR that need to be displayed on the Metavision flow sheet were also identified.
If you have any questions or concerns regarding this committee or its projects, please contact co-chairs Heidi Smith Doucette, CNRN, or Carol Booth, MSN, RN, PCCN.
Nursing Practice Committee
The Nursing Practice Committee has started the process of communicating the work of the committee to the larger nursing community throughout the Department of Nursing. The supports and conditions for excellent practice as identified by the committee members were presented and discussed at the Nurse Director Council, the Nursing Leadership Seminars and the NIC Forum. Committee members are also discussing various strategies for communicating the work of the committee to nursing colleagues in their clinical areas.
One of the supports and conditions for excellent practice identified by the committee is consistency in the nurse-patient relationship as evidenced by continuity of care. This serves as an important way to know the patient and family so they feel known and cared for. The committee had ongoing discussions about barriers as well as current practices that support continuity of care and the nurse-patient relationship.
For more information, contact committee co-chairs Mary Beth Mondello, CNRN, and Alice O’Brien, MS, RN.
Patient and Family Education Committee
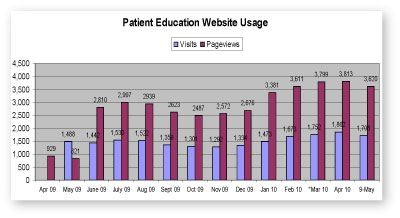
The Patient and Family Education Committee aims to make patient education resources readily available to staff nurses through the Patient Education website, now easily accessible from the Resource Button on the Pod monitor. The committee continues its efforts to increase use of the website with awareness campaigns, including the soon to be launched “Push My Buttons” campaign. Stay tuned for details.
For more information on the committee, contact Deb Moody, BSN, RN, and Cindy Loring, RNC, CNS.